How a Hidden Hand of Hepatic Signaling Could Help Fight Metabolic Disease
New research identifies PTPRD as a key regulator of metabolic liver disease – making it a potential target for treating diseases such as MASLD and MASH.
CHINA, July 21, 2025 /EINPresswire.com/ -- Chronic liver diseases tied to metabolic dysfunction—including metabolic dysfunction-associated steatotic liver disease (MASLD) and its more severe form, metabolic dysfunction-associated steatohepatitis (MASH)—represent a rising global health crisis. These diseases are fueled by poor diet, obesity, and insulin resistance, and can progress to cirrhosis and liver cancer. Despite the scale of the problem, effective treatments remain elusive. A new study published in eGastroenterology sheds light on a previously underexplored molecular player in liver health: the protein tyrosine phosphatase delta (PTPRD).PTPRD is known for its role in brain development, but its function in the liver has remained largely unexplored. The study, led by Dr. Joachim Lupberger and an international team of researchers, found that PTPRD acts as a suppressor of the pro-inflammatory STAT3 pathway in liver cells. By controlling STAT3 activity, PTPRD helps regulate lipid and glucose metabolism, protects peroxisomal function, and maintains insulin sensitivity. When PTPRD expression is reduced, these metabolic processes go awry, triggering hallmark features of metabolic liver disease.
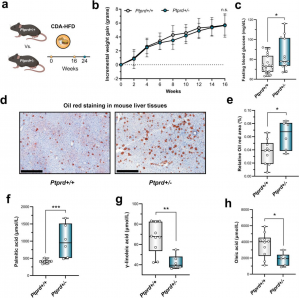
Using both human liver transcriptomic datasets and a mouse model with partial PTPRD deficiency, the team uncovered striking associations between low PTPRD expression and signs of liver dysfunction. In mice fed a high-fat, choline-deficient diet to mimic MASH, those with reduced PTPRD developed higher fasting glucose levels and significantly more liver fat than their wild-type counterparts. These animals also showed altered fatty acid profiles that resemble those in patients with diabetes and liver steatosis.
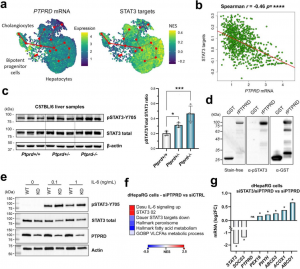
At the molecular level, the study revealed that reduced PTPRD levels lead to the overactivation of STAT3. This, in turn, suppresses genes responsible for peroxisomal lipid metabolism—a critical pathway for breaking down fatty acids. Further experiments confirmed that STAT3 is a direct substrate of PTPRD in liver cells. When PTPRD was silenced in human liver cell lines, STAT3 activity increased, and peroxisomal function decreased. Silencing both PTPRD and STAT3 reversed this effect, highlighting their direct regulatory relationship.
Notably, PTPRD deficiency also appeared to blunt insulin signaling. In primary human hepatocytes, silencing PTPRD reduced insulin-induced activation of AKT, a key mediator of insulin's metabolic effects. Transcriptomic analysis further linked low PTPRD expression to activation of the unfolded protein response (UPR), a cellular stress pathway that interferes with insulin signaling. Mice with reduced PTPRD showed increased expression of UPR-associated genes, and patients with obesity and low hepatic PTPRD had elevated markers of insulin resistance.
To validate the clinical relevance of these findings, the researchers analyzed liver biopsies and clinical data from over 700 patients with obesity. Individuals with the lowest levels of hepatic PTPRD had significantly higher blood glucose, insulin resistance scores, triglycerides, and waist circumference. These patterns matched the metabolic phenotype observed in PTPRD-deficient mice and cell models, confirming that reduced PTPRD expression is associated with worse metabolic health in humans.
The study's implications are far-reaching. First, it identifies the PTPRD-STAT3 axis as a crucial regulator of liver metabolism, offering a novel molecular target for future therapies. Unlike current treatments, which largely focus on managing symptoms or downstream effects, targeting PTPRD or its signaling pathways could directly address the underlying metabolic dysfunction. Second, it suggests that PTPRD expression levels might be a biomarker to identify individuals at higher risk for liver disease progression. Lastly, it adds to a growing body of evidence that peroxisomal and endoplasmic reticulum stress responses are central to the development of MASLD and MASH.
While the research opens exciting possibilities, the authors note that further studies are needed. The mouse model used mimics human MASH but is less suited to studying glucose metabolism and diabetes. Future work will explore PTPRD's role using alternative diet models and investigate whether restoring PTPRD function can reverse disease in advanced stages. The researchers are also examining whether components of the PTPRD protein could be detected in blood samples, potentially offering a non-invasive diagnostic tool.
Overall, this work represents a major step in understanding metabolic liver disease. By identifying PTPRD as a gatekeeper of liver metabolic health, the study opens a new chapter in the search for effective treatments. As metabolic liver diseases continue to rise worldwide, innovative approaches targeting key regulators like PTPRD may be essential to changing the course of the epidemic.
See the article:
Roca Suarez AA, Jühling F, Moehlin J, et al. Protein tyrosine phosphatase delta is a STAT3-phosphatase and suppressor of metabolic liver disease. eGastroenterology 2025;3:e100159. doi:10.1136/egastro-2024-100159
About eGastroenterology
eGastroenterology is a new, open-access, and open peer-reviewed BMJ Journal, which focuses on basic, clinical, translational, and evidence-based medicine research in all areas of gastroenterology (including hepatology, pancreatology, esophagology, and gastrointestinal surgery). eGastroenterology is now indexed by PubMed, Scopus, CAS, DOAJ, Dimensions, OpenAlex, ROAD, and COPE, with more to come!
For more information, please visit: egastroenterology.bmj.com and follow us on Twitter (@eGastro_BMJ).
Sign-up to Email Alerts for eGastroenterology: https://emails.bmj.com/k/Bmj/jausu/egastroenterology
Menghan Gao
eGastroenterology
+86 43188782545
egastro@jlu.edu.cn
Visit us on social media:
X
Distribution channels: Business & Economy, Chemical Industry, Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release