Senex Receives SNX631-6 Notice of Allowance from Japanese Patent Office

Dennis Goldberg, CEO of Senex Biotechnology
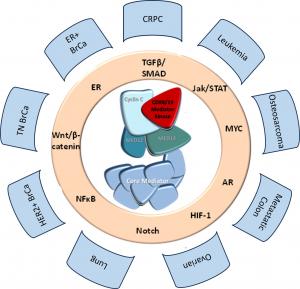
Senex Biotechnology scientists and their colleagues at the University of South Carolina have demonstrated the importance of genetic or pharmacological inhibition of CDK8 and CDK19 in multiple cancers, including all major forms of breast cancer, ovarian cancer, osteosarcoma, leukemia and metastatic colon cancer and castration resistant prostate cancer. The Company has completed IND-enabling toxicology in male animals, supported by a grant from the Department of Defense Prostate Cancer Program and will complete similar toxicology studies in female animals.
Senex was recently awarded an STTR grant for the development of a novel intravenous formulation of SNX631-6 as a treatment for metastatic ovarian cancer, and authored a manuscript published in the Proceedings of the National Academy of Sciences (PNAS) discussing the role of Mediator kinases CDK8 and CDK19 in the prevention of adaptive drug resistance and metastasis in triple negative breast cancer (TNBC). (Mediator Kinase Inhibitors Suppress Triple-Negative Breast Cancer Growth and Extend Tumor Suppression by mTOR and AKT Inhibitors , PNAS 2024 https://www.pnas.org/doi/10.1073/pnas.2414501121).
“The Japanese patent for SNX631-6 provides Senex with further global patent protection in this novel class of CDK8/19 inhibitors. The broad patent protection and our recent work in breast and ovarian cancers support Senex’s ongoing efforts in women’s health. The Company believes that SNX631-6, the Company’s clinical candidate, is the best-in-class CDK8/19 inhibitor, with low nanomolar potency, outstanding target specificity and excellent oral bioavailability. These attributes contribute to the excellent safety profile seen in our IND-enabling toxicology studies. Senex plans to initiate first in human studies in drug-resistant metastatic cancers in 2026"- Dennis I. Goldberg, Ph.D., Chief Executive Officer at Senex Biotechnology.
"Senex’s CDK8/19 inhibitors continue to show efficacy, combined with lack of toxicity, in multiple in vivo models of different cancers. These compounds are unique in showing even greater efficacy against established cancer metastases than against primary tumors, and they potentiate almost all of the tested clinical anticancer agents. I have great expectations for the transformative effects of our drug once it enters the oncological armamentarium. ”- Igor B. Roninson, Ph.D., Founder and Chief Science Officer at Senex Biotechnology.
About Senex Biotechnology
Senex Biotechnology is a drug discovery and development company focused on cancer therapeutics. Senex’s lead program targets CDK8/19, a protein that regulates gene expression and is required by cancer cells to adapt to adversarial conditions; such adaptation leads to cancer drug resistance and metastasis. Senex is developing highly selective small-molecule inhibitors of CDK8/19 for the treatment of presently incurable types of prostate cancer, breast cancer, ovarian cancer, osteosarcoma and leukemia. We are also investigating the utility of these inhibitors for different cancers in combination with other therapeutics, as well as for inflammation, cardiovascular and other diseases. Our latest, highly potent and selective drug candidate is anticipated to enter clinical trials in 2026.
Senex was founded by Dr. Igor Roninson, based on the discovery in his academic laboratory of a novel biological pathway associated with aging (senescence) and involved in cancer and other chronic diseases, as well as the use of functional genomics technologies to identify novel drug targets that are required by tumor cell but not by normal tissues. Senex has won 17 competitive grant awards from the National Institutes of Health, Department of Defense Congressionally Directed Medical Research Programs (DoD CDMRP) and the Alzheimer’s Drug Discovery Foundation. The Company’s work on breast cancer drug development was supported by a Phase I Small Business Innovation Research (SBIR) grant from the National Cancer Institute (NCI), and its work on prostate cancer was supported by a Phase II SBIR grant from the NCI and a Translational Science Award from the DoD CDMRP.
For further information, visit www.senexbio.com.
Contact information:
Dennis I Goldberg
Senex Biotechnology, Inc.
+ +1 508-878-7589
goldberg@senexbio.com
Visit us on social media:
LinkedIn
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release